Title: Validation Study of Mini-Midline Catheters for Reducing Catheter Failure Rates in Upper Arm Placement: A Randomized Controlled Trial
Journal: Drug Discoveries & Therapeutics
Author: Ryoko Murayama
Impact Factor: 3.1
Publication Date: March 2023
Objective of the Study
This study aimed to compare the incidence of catheter failure (CF) between patients receiving high-irritation medications with short peripheral intravenous catheters (PIVCs) placed in the forearm versus mini-midline catheters placed in the upper arm.
Methods
Patients from the University of Tokyo Hospital were divided into two groups: the PIVC group and the mini-midline catheter group. The primary outcome was the incidence of CF. Secondary outcomes included the rate of CF per 1,000 catheter days, catheter dwell time, and the occurrence of thrombosis and subcutaneous edema.
Results
A total of 47 patients (median age, 67.0 years) were analyzed. The CF rate in the mini-midline group was 0%, compared to 32.0% (8 catheters) in the PIVC group (p = 0.007). The CF rate per 1,000 catheter days was 0/1,000 in the mini-midline group and 81.7/1,000 in the PIVC group (p = 0.001). The difference in catheter dwell time until CF occurred was statistically significant (p = 0.004). Thrombosis and subcutaneous edema were more frequently observed in the PIVC group (p < 0.001).
Conclusion
Compared to PIVCs placed in forearm veins, mini-midline catheters placed in upper arm veins for high-irritation medications may reduce the incidence of CF.
Background
- Patients frequently use peripheral intravenous catheters (PIVCs) for intravenous infusion. However, catheter failure (CF) is common, presenting symptoms such as redness, swelling, pain, and occlusion (insufficient infusion flow), making continued drug administration difficult.
- Reported CF incidence ranges from 30% to 69%. Previous studies also indicated that about 18% of patients in adult inpatient wards at a major university hospital in Japan had catheters removed due to CF.
- The primary causes of CF are mechanical and chemical irritation of the vascular endothelial cells caused by the catheter and drugs.
- To minimize mechanical and chemical irritation, it is recommended to place the catheter in larger-diameter veins with higher blood flow. Upper arm veins are better suited for these conditions than forearm veins.
Methods
Objective:
The primary outcome was the incidence of CF. CF was defined as the unplanned removal of the catheter within 7 days (24 hours × 7 days) before the end of treatment due to signs and symptoms such as pain, swelling, redness, or occlusion, as determined by physicians or nurses. Secondary outcomes included the CF rate per 1,000 days, catheter dwell time until CF occurred, and the presence of thrombosis and subcutaneous edema.
Study Period:
August 2021 to March 2022
Study Location:
Hematology and Oncology Department, University of Tokyo Hospital
Participants:
Inclusion criteria:
Hospitalized patients aged 19 or older
Patients undergoing their first catheter insertion during hospitalization
Patients scheduled to receive high-irritation medications, such as irritants or vesicants, including chemotherapy drugs
Exclusion criteria:
Patients undergoing continuous chemotherapy infusions lasting over 24 hours
Patients with skin diseases at the planned puncture site
Patients with a history of thrombosis
Patients with chronic kidney disease (stage G3 or higher)
Patients with abnormal coagulation tests or bleeding tendencies
Patients scheduled for invasive surgery
Patients deemed unsuitable for the study by their attending physician
Procedures:
- Participants were randomly assigned to the mini-midline catheter group (New-PIVC) for upper arm veins or the PIVC group (SPC) for forearm veins.
- Mini-midline catheter: 88mm/22G, non-wire, polyurethane material, inserted under ultrasound guidance by two trained nurses.
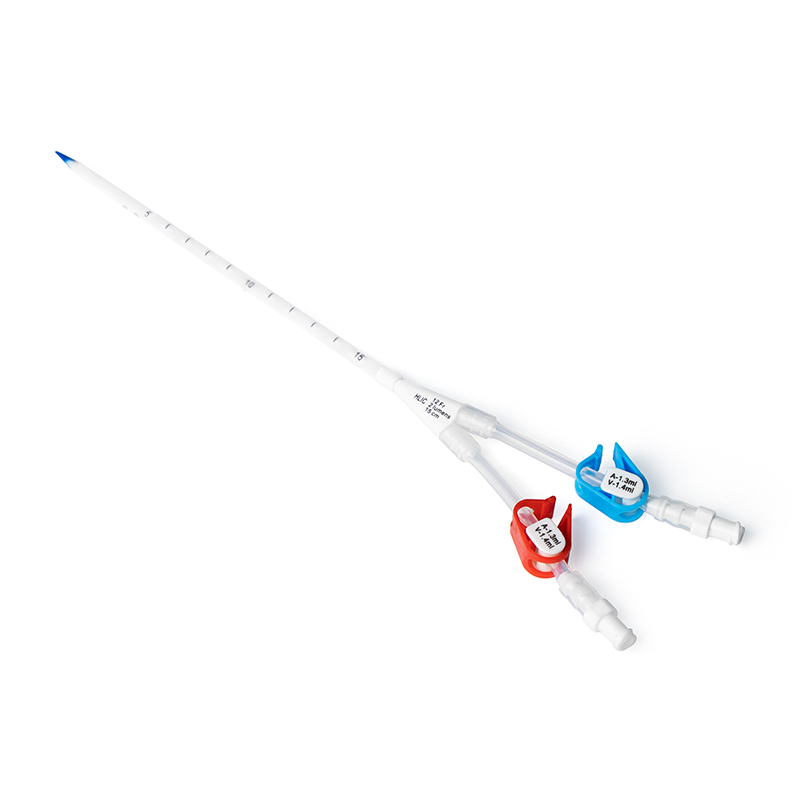
PIVC: 25mm/22G or 19mm/24G, inserted blindly by physicians.
- The protocol set a dwell time of 7 × 24 hours. On the seventh day, ultrasound was used to assess the subcutaneous tissue and vein between the puncture site and catheter tip. Venous thrombosis and subcutaneous tissue were evaluated based on previously described classifications (Figure 1). Removed catheters were photographed, and the angle of the catheter base was measured using ImageJ (NIH). Pain assessment was conducted using a Visual Analog Scale (VAS) questionnaire.

Results
- A total of 47 patients were included, with 22 in the New-PIVC group and 25 in the SPC group. Baseline characteristics were comparable between the two groups (Table 1).
.png)
2. The CF rate was 0% in the New-PIVC group and 32.0% (8 catheters) in the SPC group, with a statistically significant difference (p = 0.007).
3. The CF rate per 1,000 days was 0/1,000 days in the New-PIVC group and 81.7/1,000 days in the SPC group (p = 0.001).
4. The median dwell time for catheters in the New-PIVC group (n = 22) was 8,112.5 ± 7,045.0 minutes, compared to 4,665.0 ± 5,693.0 minutes in the SPC group (n = 25), but the difference was not statistically significant (p = 0.141) (Table 2).
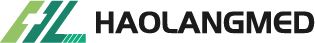




.png)