Li Xiaowen, Hu Yanling, Wan Xingli, Shi Zeyao
(Neonatal Nursing Unit, West China Second Hospital, Sichuan University / West China School of Nursing, Sichuan University; Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Chengdu 610041, China)
Abstract Objective: To retrieve the evidence related to the management of neonatal umbilical artery and umbilical vein catheters at home and abroad, and to evaluate and summarize the best practice evidence. Methods: According to the 6S model, a systematic search was conducted in domestic and foreign relevant databases such as CNKI, Wanfang, PubMed, Cochrane Library, UpToDate, and National Guideline Clearinghouse for all evidence on the management of umbilical artery and umbilical vein catheters in the neonatal intensive care unit (NICU). The retrieval time limit was from the establishment of the database to November 1, 2021. Two researchers evaluated the quality of the included literature, and extracted evidence from the literature that met the quality standards. Results: A total of 12 literatures were included, including 5 guidelines, 5 systematic reviews, and 2 clinical decisions. 18 pieces of evidence were summarized from four aspects: umbilical artery and umbilical vein catheterization-related techniques, catheter device management, catheter removal timing, and prevention of complications. Conclusion: This study provides evidence-based evidence for clinical medical staff in the NICU on the management of neonatal umbilical artery and umbilical vein catheters, promotes the standardized management of umbilical artery and umbilical vein catheters in the NICU, and reduces the incidence of related complications.
Keywords Intensive Care Units, Neonatal; Umbilical Arteries; Umbilical Veins; Evidence-Based Nursing; Best Evidence
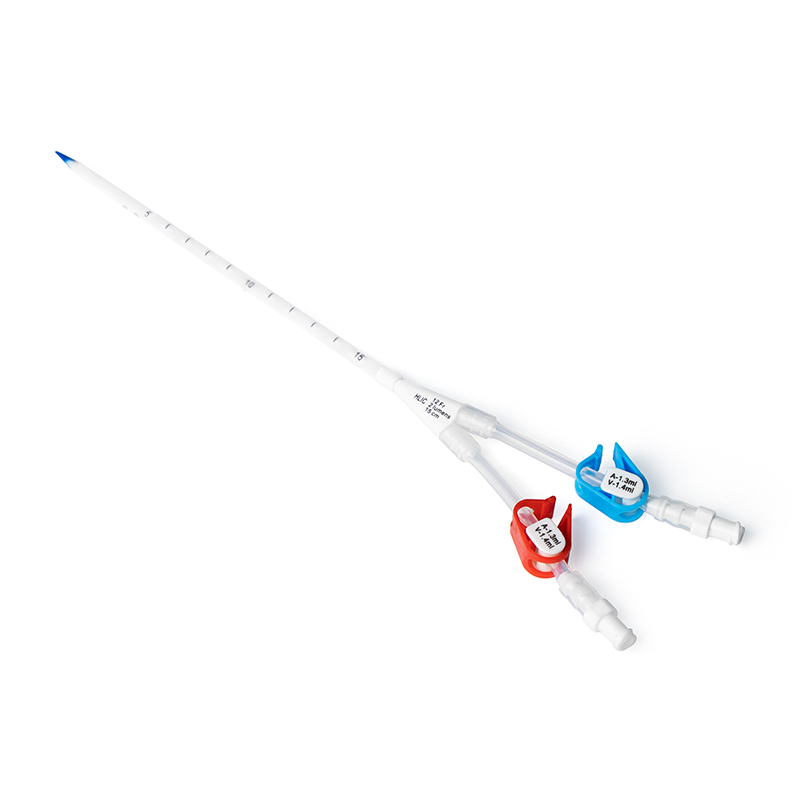
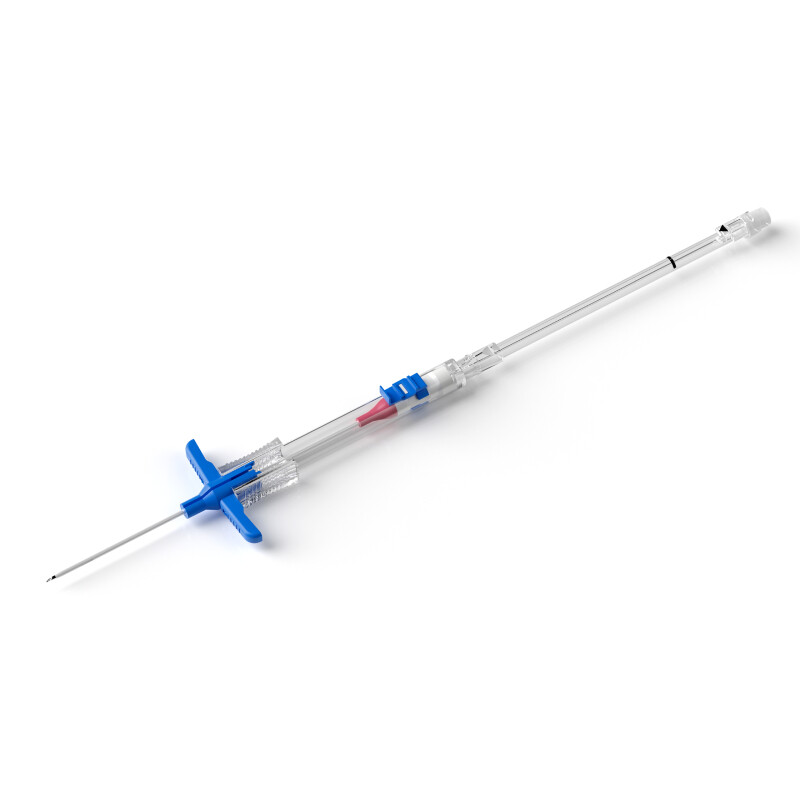
Umbilical artery and umbilical vein catheterization (umbilical vein catheter, UVC) is a very important treatment method for treating critically ill children in the neonatal intensive care unit (NICU)[1], mainly used for drug infusion, intravenous nutrition, central venous pressure monitoring, and blood exchange [2]. It has the advantages of simple operation, short time for establishing the drug administration channel, mild adverse reactions, and avoiding the pain and stimulation caused by repeated punctures to the child [3], and is an important life channel for very low birth weight infants. In addition, umbilical vein catheterization has become an important intervention in the "golden 1 hour" after the birth of premature infants. Standardized management of umbilical artery and umbilical vein catheters can improve their use safety and reduce the occurrence of serious complications such as catheter malposition, central line-associated bloodstream infection (CLABSI), and thrombosis [4-7]. At present, umbilical artery and umbilical vein catheterization has been widely carried out and applied in domestic NICUs. The best management measures related to the maintenance of umbilical artery and umbilical vein are particularly important for NICU medical staff, but there is a lack of perfect relevant guidelines or expert consensus on the maintenance and management of neonatal umbilical artery and umbilical vein in China. Therefore, this study intends to summarize the relevant evidence on the management of umbilical artery and umbilical vein catheters by Searching domestic and foreign literatures,in order to provide an evidence-based basis for the management of umbilical artery and umbilical vein catheters in NICUs, and provide a certain basis for the formulation of unified and standard relevant guidelines in the future.
1 Materials and Methods
1.1 Evidence Retrieval
The evidence-based question was constructed based on the Problem Development Tool PIPOST model of the JBI Evidence-based Health Care Center. The target population (P) for evidence application is neonates with umbilical artery or umbilical vein catheterization; the intervention (I) is the measures related to the insertion, maintenance, and related complication management of umbilical artery and umbilical vein catheters; the professionals (P) applying the evidence are neonatology medical staff; the main outcome (O) includes the incidence of complications; the setting (S) for evidence application is the neonatal intensive care unit; the type of evidence resource (T) includes clinical decisions, guidelines, expert consensus, systematic reviews, and evidence summaries.
Based on the "6S" evidence model, the search was conducted in a top-down order. The search terms for English databases were "Infant/Newborn/Neonat*", "Umbilical veins/Umbilical arteries/Umbilical venous catheter/Umbilical catheter/Central venous catheter/Intravascular catheter". Taking PubMed as an example, the search formula was: (("Infant" OR "Newborn" OR "Neonat*") AND ("Umbilical veins" OR "Umbilical arteries" OR "Umbilical venous catheter" OR "Umbilical catheter" OR "Central venous catheter" OR "Intravascular catheter")). The search terms for Chinese databases were "婴儿,新生", "脐动脉置管 / 脐动脉导管 / 脐静脉置管 / 脐静脉导管 / 中心静脉置管 / 中心静脉导管 / 血管通路 / 血管内导管". Databases such as UpToDate, BMJ Clinical Evidence, National Guideline Clearinghouse (NGC), Scottish Intercollegiate Guidelines Network (SIGN), Guidelines International Network (GIN), National Institute for Health and Clinical Excellence (NICE), Chinese Clinical Guideline Library, Embase, CINAHL, Cochrane Library, PubMed, Chinese Biomedical Literature Service System, CNKI, Wanfang Data, and VIP were searched, with the retrieval time limit from the establishment of the database to November 1, 2021.
1.2 Literature Screening Methods
Two researchers (the first author and the corresponding author) independently screened and extracted the literature according to the inclusion and exclusion criteria, and cross-checked. If there was a disagreement, it was discussed with the third researcher (the second author) to reach a consensus. The inclusion criteria for the literature were: the research object was a neonate with umbilical artery and umbilical vein catheterization; the research involved catheter maintenance; the literature types included clinical guidelines, expert consensus, evidence summaries, systematic reviews, and clinical decisions; the language was limited to Chinese and English. The exclusion criteria for the literature were: the literature types were abstracts, plans, or reports, and the literature with low quality; the literature for which complete information could not be obtained.
1.3 Evidence Quality Evaluation
- The Appraisal of Guidelines for Research and Evaluation (AGREE II) was used to evaluate the guidelines. The scale includes 6 domains (23 items) and 2 additional overall evaluation items. Each item is scored from 1 to 7 points, with 7 points indicating that the guideline fully meets the item, 2-6 points indicating that it does not fully meet the item, and 1 point indicating that it does not meet the item at all. The score of each domain is equal to the sum of the scores of all items in the domain, and is standardized to the percentage of the possible highest score of the domain. According to the standard scores of each domain, the recommendation level is divided into 3 levels: if the scores of all 6 domains are >60%, it can be recommended directly without modification; if ≥3 domains have scores of 30%-60%, it is recommended after modification and improvement to different degrees; if ≥3 domains have scores of <30%, it is not recommended.
- The assessment of multiple systematic reviews (AMSTAR) was used to evaluate the quality of systematic reviews or Meta-analyses. The scale includes 11 items, and each item has 4 options: "yes", "no", "cannot answer", and "not applicable". Selecting "yes" gets 1 point, and others get 0 points. A total score of 0-4 is low quality, 5-8 is medium quality, and 9-11 is high quality.
- For clinical decisions, the original literature included in the evidence was traced, and the quality evaluation was carried out according to the type of the original literature.
1.4 Evidence Quality Grading
The literature quality evaluation process was independently completed by two researchers who had received systematic evidence-based nursing training. If there was a disagreement, it was discussed with the third researcher to reach a consensus. When the evidence conclusions from different sources are inconsistent, the inclusion principles of evidence-based evidence priority, high-quality evidence priority, and latest published authoritative literature priority are followed. The included evidence was graded using the Australian JBI Evidence-based Health Care Center Evidence Grading System (2014). According to different research designs, the evidence is divided into levels 1 to 5 from high to low. For the evidence from the Infusion Nurses Society (INS), its evidence grading is directly applied.
2 Results
2.1 General Characteristics of the Included Literature
A total of 3,316 literatures were initially retrieved in this study. After removing 1,210 duplicate literatures, 1,723 literatures were excluded after reading the titles and abstracts, and 371 literatures were excluded after reading the full text. Finally, 12 literatures were included (Table 1). Among the 12 literatures, there were 2 clinical decisions [8-9], 5 guidelines [10-14], and 5 systematic reviews [15-19]. The detailed information is shown in Table 1.
|
First Author
|
Source
|
Nature
|
Theme
|
Publication Year
|
|
Sharon et al[8]
|
UpToDate
|
Clinical Decision
|
Vascular Access Selection for Pediatric Resuscitation and Other Emergencies
|
2020
|
|
Anthony et al[9]
|
UpToDate
|
Clinical Decision
|
Management of Thrombosis in Newborns
|
2018
|
|
The Neonatology Group of the Pediatric Society of the Chinese Medical Association, etc[10]
|
Wanfang
|
Guideline
|
Guidelines for the Prevention and Control of Complications Associated with Neonatal Umbilical Vein Catheterization
|
2021
|
|
INS[11]
|
PubMed
|
Guideline
|
Infusion Therapy Practice Guidelines
|
2016
|
|
Monagle et al[12]
|
PubMed
|
Guideline
|
Clinical Guidelines for Antithrombotic Therapy in Neonates and Children
|
2012
|
|
O'Grady et al[13]
|
National Guideline Network
|
Guideline
|
Guidelines for the Prevention of Catheter-Related Bloodstream Infections
|
2011
|
|
The Critical Care Medicine Branch of the Chinese Medical Association[14]
|
Wanfang
|
Guideline
|
Guidelines for the Prevention and Treatment of Intravascular Catheter-Related Infections
|
2008
|
|
Payne et al[15]
|
PubMed
|
Systematic Review
|
Effect of Bundled Nursing Measures on the Prevention of Catheter-Related Infections
|
2018
|
|
Taylor et al[16]
|
Cochrane Library
|
Systematic Review
|
Effect of Antibiotic Lock on the Prevention of Bloodstream Infections
|
2015
|
|
Barrington[17]
|
Cochrane Library
|
Systematic Review
|
Influence of Umbilical Artery Catheter Tip Position on the Occurrence of Complications
|
2000
|
|
Shah[18]
|
Cochrane Library
|
Systematic Review
|
Effect of Continuous Heparin Infusion on Preventing Catheter Blockage and Thrombosis in Neonatal Central Venous Catheters
|
2008
|
|
Kabra et al[19]
|
Cochrane Library
|
Systematic Review
|
Influence of Multilumen and Single-Lumen Central Venous Catheters on Neonates
|
2005
|
2.2 Results of Literature Quality Evaluation
(1) Results of guideline quality evaluation: A total of 5 guidelines were included in this study. The standardized percentage of each domain and the total quality score of the guidelines are shown in Table 2.
|
Included Literature
|
Standardized Percentage of Each Domain (%)
|
Total Quality Score of Guidelines
|
Number of Domains ≥60%
|
Number of Domains ≥30%
|
Recommendation Level
|
|
Scope and Purpose
|
Stakeholders
|
Rigor of Guideline Development
|
Clarity of Guideline Presentation
|
Applicability of Guidelines
|
Independence of Guideline Development
|
|
The Neonatology Group of the Pediatric Society of the Chinese Medical Association[10]
|
94.4
|
86.1
|
94.8
|
94.4
|
79.2
|
|
INS[11]
|
86.1
|
91.2
|
84.4
|
91.2
|
79.2
|
|
Monagle et al[12]
|
63.9
|
80.6
|
62.5
|
70.6
|
62.5
|
|
O'Grady et al[13]
|
97.2
|
91.7
|
94.8
|
97.2
|
83.3
|
|
The Critical Care Medicine Branch of the Chinese Medical Association[14]
|
69.4
|
66.7
|
62.5
|
79.4
|
56.3
|
(2) Results of systematic review quality evaluation: A total of 5 systematic reviews were included in this study. The quality evaluation results showed that 2 literatures[15,17] answered "no" to the item "whether the preliminary design plan was provided", and 1 literature[15] answered "no" to the items "whether the publication status was considered in the inclusion criteria, such as gray literature", "whether the included and excluded literatures were listed in detail", "whether the scientificity of the included studies was appropriately used in the inference of the conclusion", and "whether the possibility of publication bias was evaluated". The rest answered "yes" in the quality evaluation items.
(3) Results of clinical decision quality evaluation: The 2 included clinical decisions[8-9] traced the original literature included in the evidence, and carried out corresponding quality evaluation according to the type of the original literature, both of which were high-level evidences.
2.3 Summary and Description of Best Evidence
The relevant evidence for the maintenance of neonatal umbilical artery and umbilical vein catheters was extracted and integrated. Finally, the evidence was summarized from four aspects: catheterization-related techniques, catheter device management, catheter removal timing, and prevention of complications, forming 18 pieces of best evidence, as shown in Table 3.
3 Discussion
3.1 The Quality of the Included Literature in This Study Is High
This study aims to provide the best evidence for clinical medical staff on the management of neonatal umbilical artery and umbilical vein catheters, and has carried out strict quality evaluation on the included literature. Among the 5 included guidelines, after quality evaluation, 4 were of recommendation level A, 1 was of recommendation level B, and there was no recommendation level C. Among the 5 included systematic reviews, after quality evaluation, 4 were of high quality, only 1 was of medium quality, and there was no low-quality literature. The overall quality of the literatures included in this paper is high. This study strictly divided the level of the finally formed evidence according to the JBI evidence grading system, ensuring the applicability and scientificity of the evidence summary results. The finally summarized best evidence is marked with the source and evidence level, ensuring the authenticity and reliability of the evidence summary results. It can be seen that the best evidence content finally formed in this study can provide important reference significance for clinical nursing staff.
3.2 Strengthen the Management of Catheterization Technology and Catheter Devices
Evidence 1 to 7 summarize the key points of umbilical artery and umbilical vein catheter auxiliary catheterization, tip placement, catheterization depth, and catheter selection. Evidence 1 suggests that clinical use of real-time ultrasound guidance for catheterization should be adopted when equipment and technical conditions permit. The results of a randomized controlled trial[20] show that compared with the blind insertion X-ray positioning group, bedside real-time ultrasound-guided catheterization can reduce the total catheterization time by 46%, and the catheter malposition rate and X-ray exposure are also reduced. Evidence 2 to 3 makes suggestions on the tip positions of umbilical artery and umbilical vein catheters. The systematic review[17] results show that compared with the low-position UAC tip position (between the 4th and 5th lumbar vertebrae), the high-position UAC tip position (between the 6th and 10th thoracic vertebrae) can reduce the occurrence of clinical ischemic events, and will not cause other serious complications and sequelae. Relevant evidence suggests that the UAC should be placed in the high position as much as possible. However, the quality of the original research on the UVC tip position is not high, and high-quality research is needed for further verification in the future. Evidence 4 to 5 describes the catheter insertion depth. At present, there is a lack of unified standards for the estimation method of UVC insertion depth. Studies have proved that there is no difference in the success rate of catheterization among the commonly used catheter insertion depth formulas[Dunn method, Shukla method, modified Shukla method, surface measurement (SM) method, JSS method, birth weight method][21-23]. Therefore, the method can be selected for catheterization according to the actual situation in clinical practice. At the same time, the guideline[10] points out that when the neonatal weight is <1,000 g, the tip position of the catheter inserted by the SM method is better. Evidence 6 to 7 puts forward suggestions on the management points of catheter devices. At present, the catheter lumens are mainly single-lumen and multi-lumen. Multi-lumen catheters are mainly used for continuous drug infusion or incompatible drugs. Although studies[19] have proved that double-lumen catheters can reduce the utilization rate of peripheral venous access in neonates within 1 week after birth, which can provide certain convenience, and the incidence of double-lumen UVC catheter failure is not higher than that of single-lumen catheters, the use of multi-lumen catheters may increase medical nursing operations, thereby increasing the risk of CLABSI. Therefore, it is still recommended to choose single-lumen catheters clinically to avoid serious complications such as infection. In addition, since existing studies cannot compare the effects of different antibiotic lock solutions on preventing CLABSI, routine use of antibiotic locks for catheter sealing is not recommended in clinical practice.
3.3 Strictly Grasp the Timing of Catheter Removal
Evidence 8 to 12 explains the indwelling time of umbilical artery and umbilical vein and the timing when the catheter should be removed. Evidence 8 to 9 describes the maximum indwelling time of umbilical artery and umbilical vein catheters. At present, the indwelling time of neonatal UVC at home and abroad is inconsistent. Foreign guidelines[13] suggest that when the aseptic management is proper, the indwelling time of umbilical vein catheters can be extended to 14 days; however, domestic relevant guidelines[10] propose that the indwell